What Is Delta Tf in Chemistry
100 2 ratings in chemistryit stands. 475 4119 Views.

Solved Colligative Properties Are Directly Related To The Chegg Com
Since Kf is a constant or a number that is always the same it is often provided in a chart or table in chemistry books.

. How do you calculate TF in chemistry. Delta Tf Kfcm where cm is the molal concentration of the solution. DS qt when youre dealing with a reversible process.
To calculate the freezing point depression constant or Kf youll need the equation. The freezing point Tf of a solution of a nonvolatile solute is lessthan that of the pure solvent. What is the molal freezing point depression constant K f of benzene.
The change in freezing point DeltaTf also called the freezing point depression Td is given by. Since Kf is a continuing or a quantity thats all the time the identical its typically offered in a chart or desk in chemistry books. What is the freezing point.
In chemistry it means increase. 33 Votes To calculate the freezing point depression constant or Kf youll need the equation. We at Merus use it for example to measure the efficiency of a heat exchanger.
Any delta is always the final f value minus the initial i value. To calculate the freezing level melancholy fixed or Kf you will want the equation. The boiling point of an aqueous solution is 10248 C.
It is used to find out how long it takes to get from one place to another. High evaporator Delta T means that the incoming temperature and outgoing temperature is excessively large. The second law states that there exists a useful state variable called entropy.
Delta Tf Kfcm the place cm is the molal focus of the answer. In the world of chemistry S is the symbol for entropy. 160 g of naphthalene C 10 H 8 is dissolved in 200 g of benzeneThe freezing point of pure benzene is 55 o C and the freezing point of the mixture is 28 o C.
What does delta Tf stand for in chemistry. The change in entropy delta S is equal to the heat transfer delta Q divided by the temperature T. The full equation is Δt tf - ti where delta the triangle represents the time interval.
What is Delta TF in chemistry. This is true for any solute added to a solvent the freezing point of the solution will be lower than the freezing point of the pure solvent. Write down what you understand.
Given that Δ Tf is the depression in freezing point of the solvent in a solution of a non - volatile non - electrolytic solute of molality m then quantity - limit m0 Δ Tfm is equal to molal depression constant. A dirty air filter. The symbol means heat in the chemical reaction.
Calculate the freezing point depression of benzene. Delta Tf Kfcm where cm is the molal concentration of the solution. I Keshav you are absolutely right molarity equal to moles upon volume.
Delta T Kbm Add to normal boiling point. I know that formula for freezing point is delta Tf Kfm but what do I plug in for each. Delta T Tf - Ti.
Tf Freezing point of pure solvent Freezing point of solution. It follows from this that Delta S is the change in the entropy of a given system and surroundings. I have explained you in one question of yours but where moles upon mass of solvent is written its molality.
The change in freezing point DeltaTf also called the freezing point depression Td is given byDelta Tf Tf of pure solvent - Tf of solutionStudies have shown thatDelta Tf also equals Kf molality of solutionwhere Kf is the freezing point depression. Delta Tf Tf of pure solvent - Tf of solution Studies have shown that. Calculate the freezing point depression of benzene.
So the change in temperature delta T is equal to the final temperature Tf minus the initial temperature Ti. This is the best answer based on feedback and ratings. This would be written as.
How do you calculate TF in chemistry. Solve Study Textbooks Guides. The change in freezing point DeltaTf also called the freezing point depression Td is given by.
Its usually caused by low air flow across the coil which includes problems like. Log in or register to post comments. Write down what you know.
Also in case you didnt know this scientists use the delta symbol to denote a change in a quantity. What is Gibbs energy class 11. Class-12-science Chemistry.
Chemistry The freezing point Tf of a solution of a nonvolatile solute is lessthan that of the pure solvent. Calculate the freezing point depression of benzene. What is Delta TF in chemistry.
In mathematics the capital delta Δ means change In the case of X representing the speed of an object and if accompanied by delta Δx it refers to change in speed. For a given physical process the entropy of the system and the environment will remain a constant if the process can be reversed. A triangle in a chemical reaction is the capital Greek symbol delta Δ.
The freezing point Tf of a solution of a nonvolatile solute is lessthan that of the pure solvent. AP Chemistry Johns Hopkins University Center For Talented Youth Lab Information Freezing Point Depression Calculations ΔT f iK f m ΔT f is the freezing point depression decrease in freezing point i The vant Hoff factor accounts for dissociation of solute in solution. Delta Tf also equals Kf molality of solution.
Delta T Kfm Subtract from normal freezing point. Fan set to an incorrect speed. The temperature differs either in time andor position.
Delta Tf Tf of pure solvent Tf of solution. The term Delta T ΔT is in science the difference of temperatures between two measuring points. Calculate the expected freezing.
Your C value will change based on whether your volume Cv or pressure Cp are constant. DS nRln VfVi when the process is isothermal. DS nCln TfTi when theres a change in temperature associated with the change in entropy.
The reversible work in thermodynamics implies a special method in. Gibbs Energy is the maximum or reversible work that a thermodynamic system can perform at a constant temperature and pressure. For this experiment the i 1.
The delta symbol entered the general practice of using the symbol after the works of Leonard Euler in 1755. The freezing point depression is the difference in the freezing points of the solution from the pure solvent. Delta S delta q T.
What the formula for delta Tf in terms of Tf and T Share with your friends.
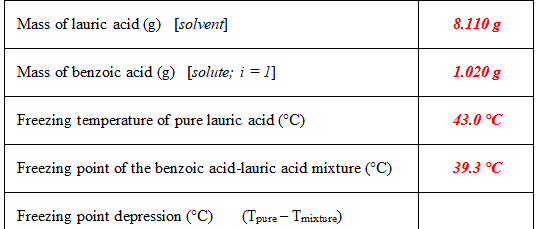
Solved The Freezing Point Depression Is 3 7 Degrees C 1 Chegg Com

The Molecular Nature Of Matter And Change Ppt Download

Chemistry 4 2 Colligative Properties Flashcards Quizlet

Answered General Chemistry Ii Laboratory Manual Bartleby
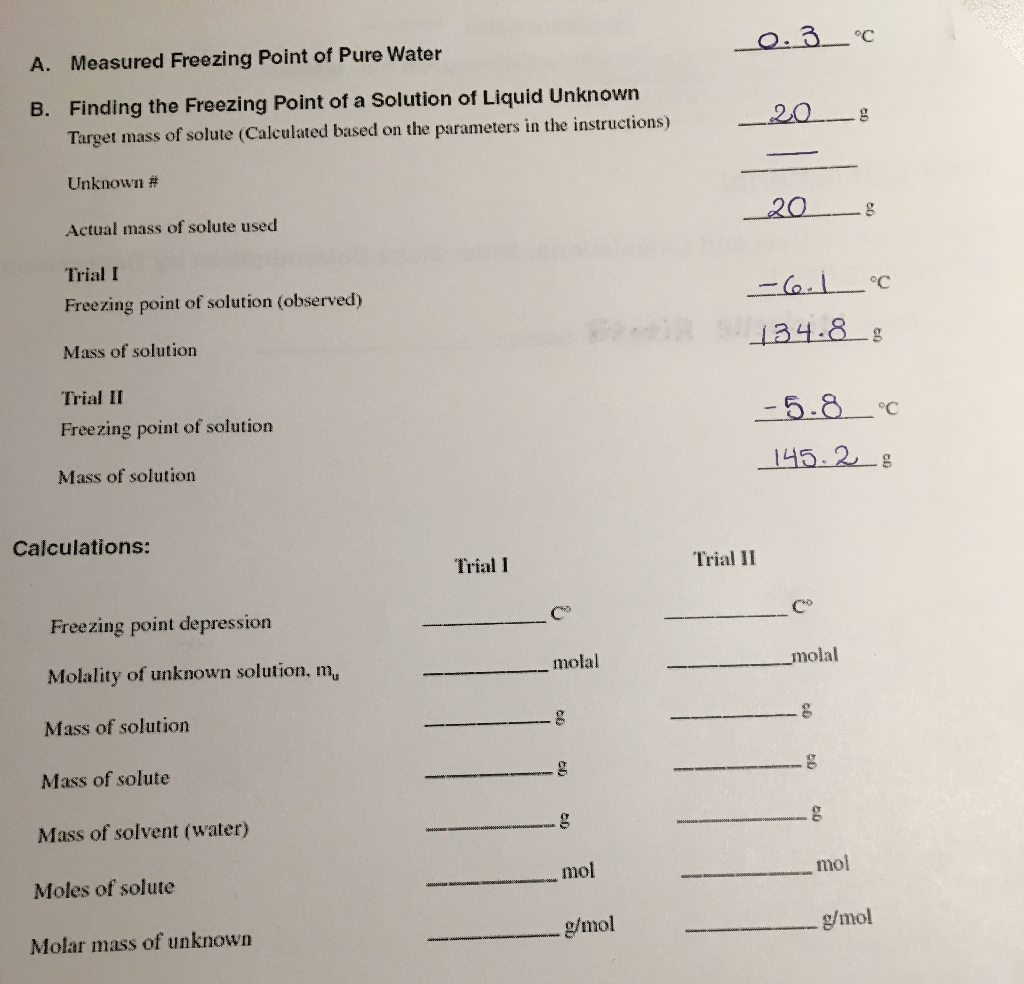
Solved How Would I Go About Solving For The Questions In Chegg Com

Aqueous Solution Of Barium Phosphate Which Is 100 Ionised Has Delta Tf Kf As 0 05 Hence Chemistry Solutions 13021661 Meritnation Com
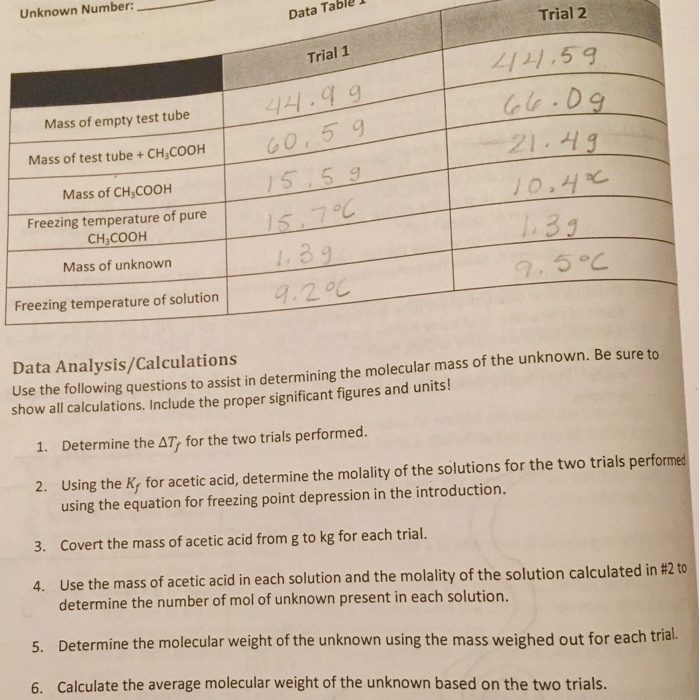
Solved Trial 1 And Trial 2 Chegg Com

How Is Of Delta Tf And Delta Tb Related To Molecular Mass Of Solute Brainly In
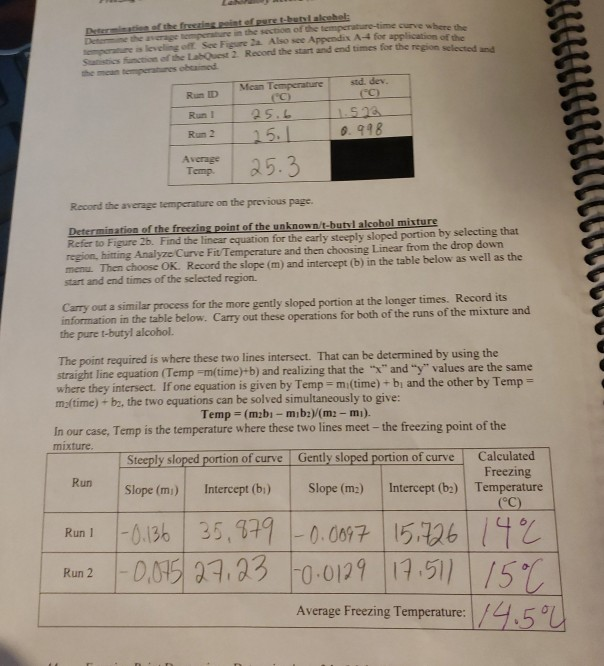
Solved Find 9 Delta Tf Between T Butyl Alcohol And Chegg Com

Write A Short Note On Depression Of Freezing Point Chemistry Solutions 11535006 Meritnation Com

Chem 112 Lab Lab 6 Freezing Point Depression Flashcards Quizlet

Here Is All Of The Known Information I Need Help Chegg Com

How Is Of Delta Tf And Delta Tb Related To Molecular Mass Of Solute Chemistry Some Basic Concepts Of Chemistry 12800339 Meritnation Com

Solved What Is The Correct Delta Tf And How Do I Calculate Chegg Com

Solved Question 3 10 Points What Does Delta Tf D Tf Chegg Com

The Molecular Nature Of Matter And Change Ppt Download

Solved Define Boiling Point Elevation And Freezing Point Depression Write The Equations Relating Boilingpoint Elevation And Freezing Point Depression To The Concentration Of The Solution Define All The Terms And Give Their Units

